THE PROJECT
Technological Innovation In Healthcare
The National Heart, Lung, and Blood Institute (NHLBI) within the National Institutes of Health (NIH) Small Business Innovation Research (SBIR) Program aims to foster technological innovation and develop groundbreaking solutions that address crucial health challenges. This program promotes collaboration between small businesses, research institutions, and the biomedical industry, with the goal of expediting the commercialization of inventive technologies.
The NIH grant review process, which includes a peer review and scientific review, evaluates grant applications submitted in response to a funding opportunity announcement. NIH staff and a review group, consisting of experts in the field, assess the applications based on their scientific merit and potential impact on health. Applicants receive guidance from the institute or center they apply to, and submit their proposals through the eRA Commons system, which provides a secure platform for managing the application and review process.
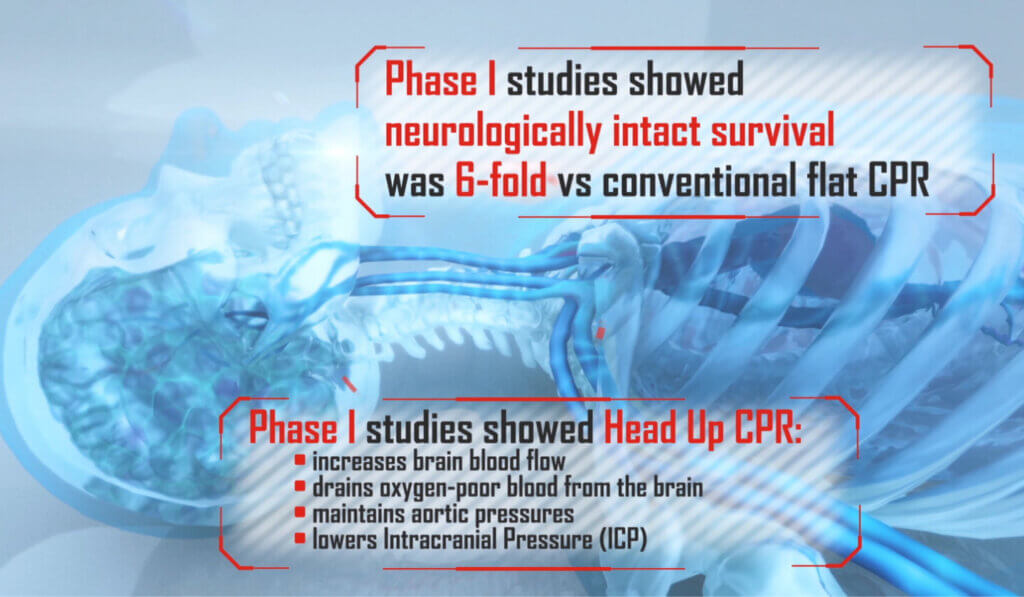
AdvancedCPR Solutions® received a Phase 1 NIH grant after successfully navigating the NIH grant review process, with their proposal to develop a medical device using the ElevatedCPR method. This innovative approach raises the head, heart, and thorax during Cardiopulmonary Resuscitation (CPR). The results demonstrated that the EleGARD™ Patient Positioning System led to a sixfold increase in neurologically intact survival rates compared to conventional CPR.
THE CHALLENGE
Video Development And Medical Device Productization
KAIA was tasked with two primary objectives:
- Creating a persuasive video to support the pursuit of Phase II funding from the NIH grant, following the funding opportunity announcement.
- Front-end medical device development, increased user-friendly and for emergency response and medical professionals.
THE SOLUTION
Persuasive Video For Phase II NIH Grant Application
To address the challenge of creating a persuasive video supporting the pursuit of Phase II funding from the NIH grant, KAIA developed a visual strategy that effectively communicated the life-saving potential of this advanced medical technology. This strategy was in line with the NIH grant review process, which includes a peer review, a scientific review, and adherence to the guidelines set forth in the funding opportunity announcement.
The video incorporated scientific principles underlying the technology and highlighted the potential impact of this innovative device on improving survival rates and neurological outcomes in SCA victims. KAIA used clear and engaging visuals and narratives, along with compelling statistics on the high incidence of SCA and the low survival rate of SCA victims, to effectively communicate the urgency and importance of developing innovative solutions to address this critical health challenge. Additionally, it presented an overview, summary, and related information on the EleGARD™ system, showcasing its potential to revolutionize CPR administration during sudden cardiac arrest.
Streamlined Medical Device Development Process
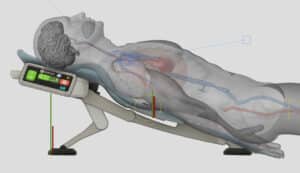
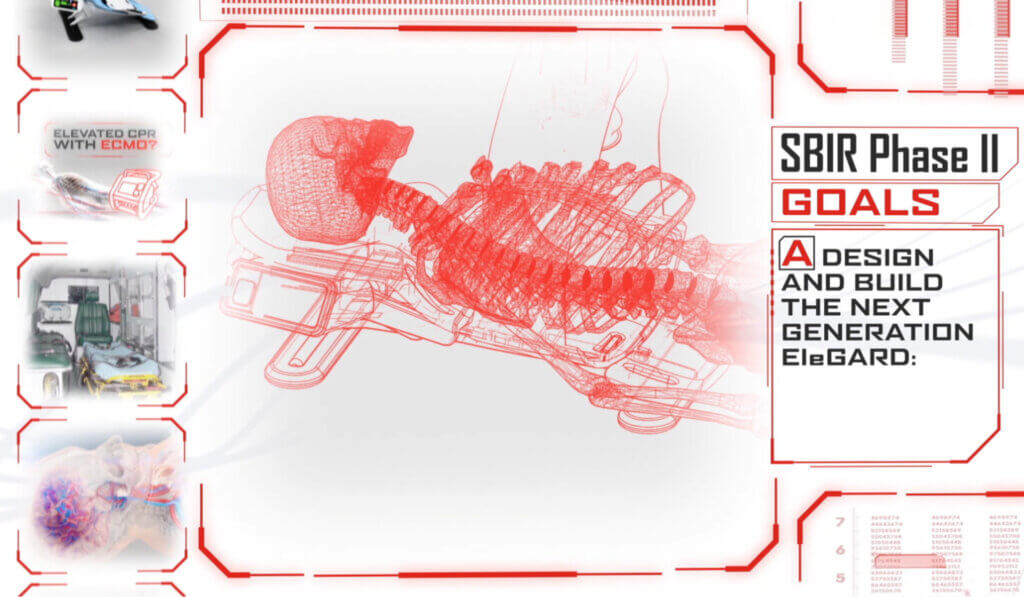
To optimize the design, development, and production processes, KAIA implemented productization strategies that focused on creating a more compact, portable, and user-friendly version of the device. Balancing the device’s size, weight, and functionality, while ensuring its safety and efficacy, was a complex process. Furthermore, considerations such as low volume production, price reduction, and cleanability were taken into account during productization.
Throughout the medical device development process, KAIA worked closely with key stakeholders to address user needs and ensure that the final product adhered to good manufacturing practices, as well as the legislative aspects of the medical device regulations. By effectively balancing the competing demands of size, portability, safety, efficacy, production, price, and cleanability, the EleGARD™ device has the potential to become a viable and competitive product that meets the needs of healthcare professionals and improves the survival rates of sudden cardiac arrest victims.


THIS PROJECT INCLUDED:
- Product Design
- Graphic Support
- Video Production
- Brand Storytelling
THE OUTCOME
Phase II Grant
Thanks to the compelling video and optimized medical device design, AdvancedCPR Solutions® received a $1.99 million Phase II grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health Small Business Innovation Research Program. This funding supports the company’s ongoing research and development, advancing the project and bringing it closer to becoming a viable and competitive medical product that meets the needs of healthcare professionals and improves the survival rates of sudden cardiac arrest victims.
KAIA can help you with digital and physical product development, as well as wrapping the work in compelling visuals and engaging story that explains the value it delivers.


